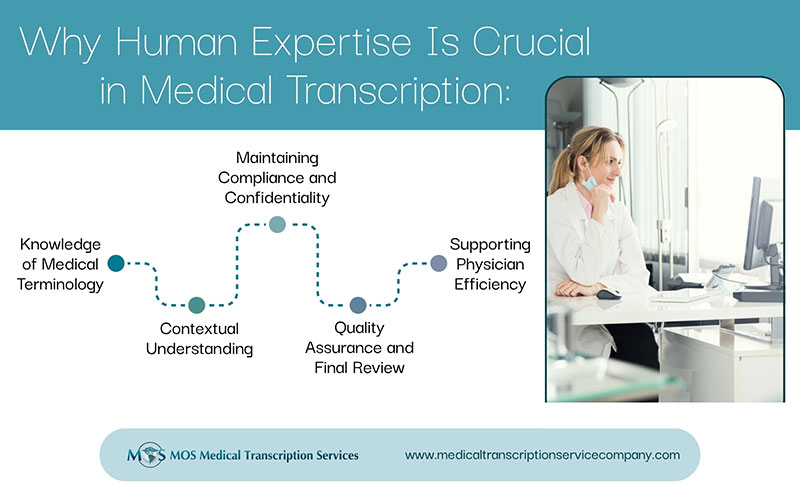
Retained surgical items are a major concern for infection prevention and surgical services departments. In hospital settings, incidents of unintended retention of foreign objects occur in operating rooms, labor and delivery areas, ambulatory surgery centers, and other areas where invasive procedures are performed. Good evidence-based processes for preventing retained objects are as crucial as the quality operation notes that medical transcription companies provide.
Magnitude of the Problem
Retained surgical items were ranked eighth on the ECRI Institute’s 2016 Top 10 Patient Safety Concerns list. The Joint Commission received 772 voluntary reports of retained surgical items from 2005-12, while other research found that 4,500 to 6,000 surgical item cases occur annually in the U.S. (Becker’s Hospital Review). According to the Association of periOperative Registered Nurses (AORN), the most common inadvertently retained items are:
- Soft goods, such as sponges and towels
- Small miscellaneous items, including unretrieved device components or fragments (such as broken parts of instruments), stapler components, parts of laparoscopic trocars, guidewires, catheters, and pieces of drains
- Needles and other sharp implements
- Instruments, most commonly malleable retractors
Risks to the patient depend on what the item is, where it is in the patient and how long it has been there. Complications result when the retained item leads to infection or causes damage to surrounding tissue. Surgical cotton gauze sponges are the most harmful retained surgical item because additional surgery is usually required for removal (Becker’s Hospital Review).
In addition to posing a serious health threat for patients, unintentionally retained surgical items can cause financial and reputational damage for organizations. In February 2019, The Sacramento Bee reported that the California Department of Public Health announced that regulators were fining Redding’s Mercy Medical Center after a patient died due to chest inflammation caused by a medical sponge left behind during surgery.
Root Causes of Unintended Surgical Items
The main reasons for unintended surgical items are:
- lack of policies and procedures
- unreliable practices such as inconsistency when counting sponges
- noncompliance with existing policies and procedures
- failures in communication with surgeons
- nurses not communicating relevant patient information
- problems with hierarchy and intimidation
- inadequate or incomplete education of staff
According to a 2017 study about 88 percent of retained surgical item cases happened during a procedure in which sponge and instrument counts were declared “correct,” which suggests human error.
Risks for unintended retained items include: patients with high body mass index, an emergency procedure, and unexpected intraoperative development. Other risk factors include more than one surgical procedure, multiple surgical teams, and multiple staff turnovers during the procedure.
Prevention Measures
Experts recommend several measures to prevent retained surgical items. The recommendations of the AORN Guideline for Prevention of Retained Surgical Items are as follows:
- A consistent multidisciplinary approach that includes standardized, transparent, verifiable and reliable prevention measures
- Accounting for instruments, surgical soft goods, sharps and other miscellaneous items opened onto the sterile field during all procedures in which these items are used
- Taking measures to prevent device fragments
- Taking standardized measures for reconciling count discrepancies during the closing count and before the end of surgery. If there is a discrepancy in a count, the surgical team should take actions to find the missing item
- Evaluation of adjunct technologies for use as a supplement to manual counting procedures
- Readily available documentation to reflect activities related to prevention of RSIs
- Development of policies and procedures for the prevention of RSIs, with periodical review and revision as necessary
- Participation of perioperative personnel in quality assurance and performance improvement activities
The Joint Commission recommends that a counting procedure should be performed audibly and visibly by two persons engaged in the process. The surgical team should verbally acknowledge verification of the count. The count should be performed before the procedure begins, in order to establish a baseline count; before the closure of a cavity within a cavity; before wound closure begins; at skin closure or end of procedure; and at the time of permanent relief of either the scrub person or the circulating registered nurse.
A July 2019 article in Outpatient Surgery (XX, No. 7) titled “Technology to Prevent Retained Items” provides several examples of sponge-detection technology that can protect against retained items. These include:
- Matrix label or barcoding – using each sponge’s unique identifying number, this program tracks sponges counted in and out.
- Radio frequency detection device – all the sponges for a case come with a small tag that can align with a radio frequency device. The detection wand can detect the tagged sponges if they are left in a patient or put in the trash.
- Combined approach – In this system, each sponge features its own unique RFID transmitter that can be detected by a wand device and scanned into and out of a program on a touch-screen table. It can identify the exact location, type and number of sponges in the surgical site.
As counting is prone to error, standardized counting practices are obviously the best way to make certain that all surgical items are accounted for and recorded. New technology can go a long way in preventing counting errors and ensuring that nothing is left behind.
A crucial aspect of documentation for surgical procedures, quality operation notes are essential for patient safety, post-operative care, remuneration and defense in litigation. Partnering with an experienced medical transcription services company can help providers ensure proficient, legible operation notes.